REAL TIME RT-PCR ASSAY TO DETECT SARS-COV-2 IN THAILAND
DOI:
https://doi.org/10.55374/jseamed.v5i1.77Keywords:
Real time RT-PCR, COVID-19, SARS-CoV-2Abstract
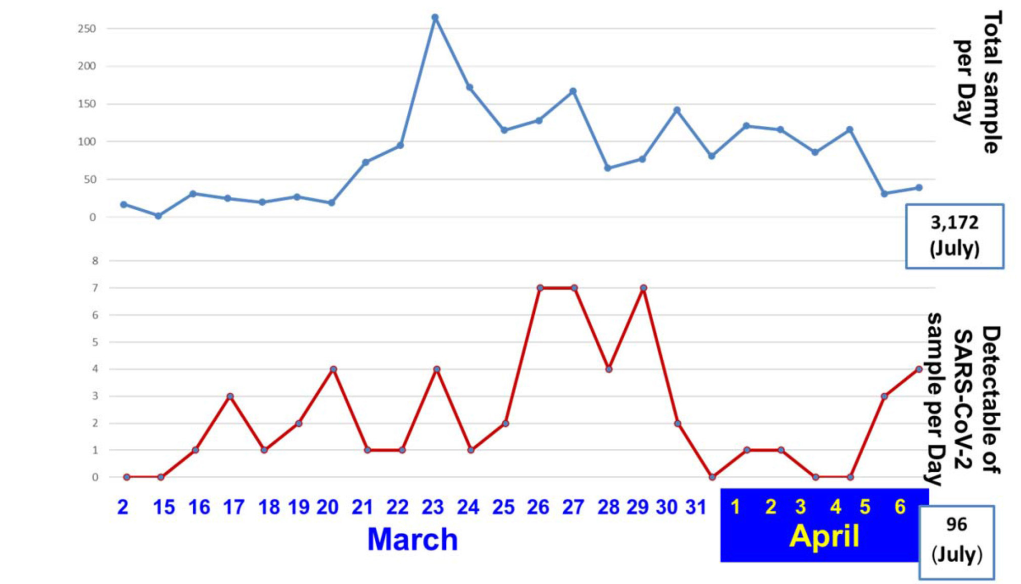
The Armed Forces Research Institute of Medical Sciences (AFRIMS) conducts medical research and disease surveillance to develop and evaluate medical products, vaccines and diagnostics to protect Royal Thai Army personnel from infectious diseases. Currently regarding globalized travel, infectious diseases pose a constantly evolving threat, indiscriminately transcending national, regional and even intercontinental boundaries. Since the COVID-19 outbreak, AFRIMS has gained knowledge from diagnostic tests for SARS-CoV-2 using the Centers for Disease Control and Prevention (CDC) as a reference protocol. We set up and developed the molecular diagnosis detection for SARS-CoV-2, Real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR), to analyze the nucleocapsid (N) genome of SARS-CoV-2 which is a standardized method in the laboratory. AFRIMS is certified by the Department of Medical Science, Ministry of Public Health (MOPH) as a COVID-19 laboratory network. We provided COVID-19 screening services to government units and private hospitals in late February 2020. It could be stated that AFRIMS is the first military unit to be certified by the MOPH. Since the COVID-19 pandemic started in Bangkok, 3,172 samples have been tested and 96 samples have been confirmed. Detecting viral RNA not only aids in the diagnosis of illness but also provides epidemiological and surveillance information.
Downloads
Metrics
References
Gao GF. From “A” IV to “Z” IKV: attacks from emerging and re-emerging pathogens. Cell 2018; 172: 1157-9. DOI: https://doi.org/10.1016/j.cell.2018.02.025
Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 2011; 81: 85-164. DOI: https://doi.org/10.1016/B978-0-12-385885-6.00009-2
Masters PS, Perlman S. Coronaviridae. In: Knipe DM, Howley PM, eds. Fields virology. 6th ed. Lippincott Williams & Wilkins, 2013: 825-58.
Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016; 24: 490-502. DOI: https://doi.org/10.1016/j.tim.2016.03.003
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019; 17: 181-92. DOI: https://doi.org/10.1038/s41579-018-0118-9
Zhong NS, Zheng BJ, Li YM, Poon LLM, Xie ZH, Chan KH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet 2003; 362: 1353-8. DOI: https://doi.org/10.1016/S0140-6736(03)14630-2
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1953-66. DOI: https://doi.org/10.1056/NEJMoa030781
Drosten C, Günther S, Preiser W, Werf SVD, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967- 76. DOI: https://doi.org/10.1056/NEJMoa030747
Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367: 1814-20. DOI: https://doi.org/10.1056/NEJMoa1211721
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW et al, Diagnostic detection of 2019-nCoV by real-time RT-PCR. Eurosurveillance 25(3), DOI: 10.2807/1560- 7917.ES.2020.25.3.2000045 DOI: https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
Zhou Wang, Wang Qiang and Hu Ke. A Handbook of 2019 nCoV Pneumonia Control and Prevention. Hubei Science and Technology Press
Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus by Centers for Disease Control and Prevention (CDC) Effective: 03/30/2020
Downloads
Published
How to Cite
Issue
Section
License
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







