Renal Injury and Dysfunction among HIV Positive Patients Receiving Tenofovir Based Anti-Retroviral Therapy
DOI:
https://doi.org/10.55374/jseamed.v1i1.34Keywords:
Tenofovir, Tubulopathy, Acute kidney injuryAbstract
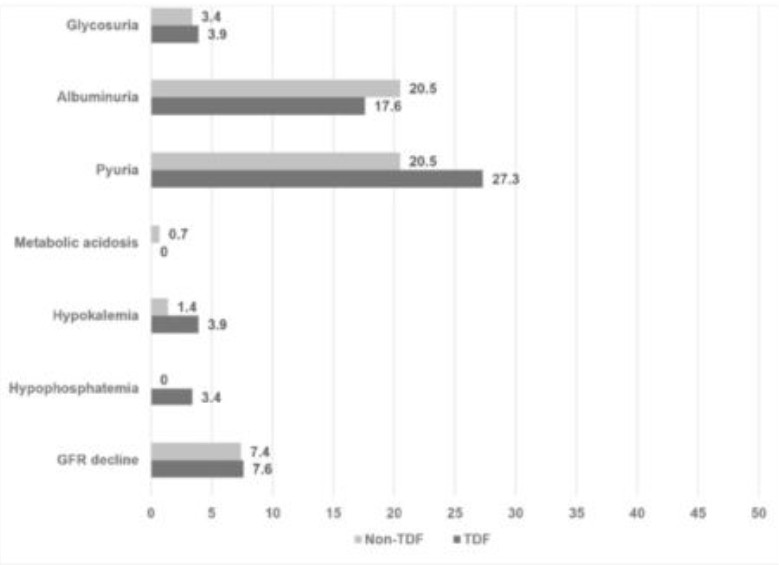
The rate of renal disease among patients with HIV has decreased significantly since the introduction of highly active antiretroviral therapy (HAART). Patients receiving tenofovir, disoproxil, fumarate (TDF) had an increased prevalence of proximal renal tubular dysfunction and injury but its clinical significance remain controversial. To difine the renal tubulopathy injury among patients with HIV with and without TDF. A cross-sectional study was conducted among HIV positive patients receiving TDF (N= 176) and non TDF regimen (N= 146) at outpatient clinic. All patients were evaluated regarding serum creatinine, electrolytes, phosphate and differing urinary parameters (proteinuria, glycosuria and pyuria). Estimated glomerular filtration rate (GFR) was calculated using CKD-EPI equation. Of 322 participants with mean age of 41.6+-11.4 years and HIV duration of 7.2+-4.3 years, the TDF and non TDF groups were similar on most clinical and demographic factors. GFR was 100.6+- 17.8 mL/min/1.73 m2 in TDF group and 97.5+- 19.6 mL/min/1.73 m2 in non-TDF group (p= 0.143). During evaluation, 3.4% of TDF patients vs. none of the non TDF-patients had hypophosphataemia (< 2.5 mg/dL), 3.9% of TDF-patients vs. 1.3% of non TDF had hypokalemia (< 3.5 mg/dL), and 0.68% of TDF-patients vs. none of non TDF patients had acidosis (< 18 mEq/L) with no statistically significant difference between groups. The proportion of patients with evidence of urine abnormalities was also similar in the two groups (Dipstick proteinuria > 1+, TDF: 17.6% vs. non-TDF 20.5%, p= 0.568, and pyuria; TDF: 27.3% vs. non TDF 20.5%, p= 0.192). Renal impairment, electrolyte disturbances and renal tubulopathy were uncommon among HIV positive patients receiving TDF-based antiretroviral therapy and did not significantly differ between TDF and non TDF regimens
Downloads
Metrics
References
Malik A, Abraham P, Malik N. Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment--case report and review of literature. J Infect. 2005;51(2):E61-5. DOI: https://doi.org/10.1016/j.jinf.2004.08.031
Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40(6):1331-3. DOI: https://doi.org/10.1053/ajkd.2002.36924
Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-75. DOI: https://doi.org/10.1093/cid/cis937
Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40(8):1194-8. DOI: https://doi.org/10.1086/428840
Gallant JE, Winston JA, DeJesus E, Pozniak AL, Chen SS, Cheng AK, et al. The 3-year renal safety of a tenofovir disoproxil fumarate vs. a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS. 2008;22(16):2155-63. DOI: https://doi.org/10.1097/QAD.0b013e3283112b8e
Reid A, Stohr W, Walker AS, Williams IG, Kityo C, Hughes P, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46(8):1271-81. DOI: https://doi.org/10.1086/533468
Madeddu G, Bonfanti P, De Socio GV, Carradori S, Grosso C, Marconi P, et al. Tenofovir renal safety in HIV-infected patients: results from the SCOLTA Project. Biomed Pharmacother. 2008;62(1):6-11. DOI: https://doi.org/10.1016/j.biopha.2007.04.008
Nishijima T, Komatsu H, Gatanaga H, Aoki T, Watanabe K, Kinai E, et al. Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PLoS One. 2011;6(7):e22661. DOI: https://doi.org/10.1371/journal.pone.0022661
Gupta SK, Anderson AM, Ebrahimi R, Fralich T, Graham H, Scharen-Guivel V, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS One. 2014;9(3):e92717. DOI: https://doi.org/10.1371/journal.pone.0092717
Stohr W, Reid A, Walker AS, Ssali F, Munderi P, Mambule I, et al. Glomerular dysfunction and associated risk factors over 4-5 years following antiretroviral therapy initiation in Africa. Antivir Ther. 2011;16(7):1011-20. DOI: https://doi.org/10.3851/IMP1832
Mulenga L, Musonda P, Mwango A, Vinikoor MJ, Davies MA, Mweemba A, et al. Effect of baseline renal function on tenofovir-containing antiretroviral therapy outcomes in Zambia. Clin Infect Dis. 2014;58(10):1473-80. DOI: https://doi.org/10.1093/cid/ciu117
Cao Y, Han Y, Xie J, Cui Q, Zhang L, Li Y, et al. Impact of a tenofovir disoproxil fumarate plus ritonavir-boosted protease inhibitor-based regimen on renal function in HIV-infected individuals: a prospective, multicenter study. BMC Infect Dis. 2013;13:301. DOI: https://doi.org/10.1186/1471-2334-13-301
Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496-505. DOI: https://doi.org/10.1086/655681
Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867-75. DOI: https://doi.org/10.1097/QAD.0b013e328351f68f
Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78(11):1171-7. DOI: https://doi.org/10.1038/ki.2010.318
Perazella MA. Tenofovir-induced kidney disease: an acquired renal tubular mitochondriopathy. Kidney Int. 2010;78(11):1060-3. DOI: https://doi.org/10.1038/ki.2010.344
Cheng CY, Chang SY, Lin MH, Ku SY, Sun NL, Cheng SH. Tenofovir disoproxil fumarate-associated hypophosphatemia as determined by fractional excretion of filtered phosphate in HIV-infected patients. J Infect Chemother. 2016;22(11):744-7. DOI: https://doi.org/10.1016/j.jiac.2016.08.008
Haverkort ME, van der Spek BW, Lips P, Slieker WA, ter Heine R, Huitema AD, et al. Tenofovir-induced Fanconi syndrome and osteomalacia in two HIV-infected patients: role of intracellular tenofovir diphosphate levels and review of the literature. Scand J Infect Dis. 2011;43(10):821-6. DOI: https://doi.org/10.3109/00365548.2011.577805
Day SL, Leake Date HA, Bannister A, Hankins M, Fisher M. Serum hypophosphatemia in tenofovir disoproxil fumarate recipients is multifactorial in origin, questioning the utility of its monitoring in clinical practice. J Acquir Immune Defic Syndr. 2005;38(3):301-4
Downloads
Published
How to Cite
Issue
Section
License
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







