PREVALENCE OF METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS AND OTHER STAPHYLOCOCCAL NASAL CARRIAGES AMONG HEALTHCARE WORKERS, PHRAMONGKUTKLAO HOSPITAL
DOI:
https://doi.org/10.55374/jseamed.v6i0.122Keywords:
MRSA, MALDI-TOF MS, Staphylococcus epidermidis, MSSA, mecA gene, SCCmecAbstract
Background: Methicillin-resistant Staphylococcus aureus (MRSA) is a group of S. aureus strains containing the SCCmec gene causing beta-lactam antibiotic resistance. MRSA is common in healthcare settings and can cause serious problems.
Objective: The study aimed to investigate the prevalence of MRSA nasal colonization among privates of the Medical Private Company, Phramongkutklao Hospital, including antibiotic susceptibility pattern of S. aureus isolates and risk factors of S. aureus nasal carriage.
Methods: Nasal swabs were obtained from the anterior nares of 170 privates. Staphylococcal isolates were identified using a catalase test, tube coagulase test and matrix-assisted laser desorption/ ionization time of flight mass spectrometry (MALDI-TOF MS). MRSA detection was screened using cefoxitin disk diffusion and confirmed using the mecA gene detection and SCCmec typing. Antibiotic susceptibility patterns of S. aureus were examined using the disk diffusion method. A questionnaire was collected from the subjects to determine risk factors for S. aureus nasal carriage.
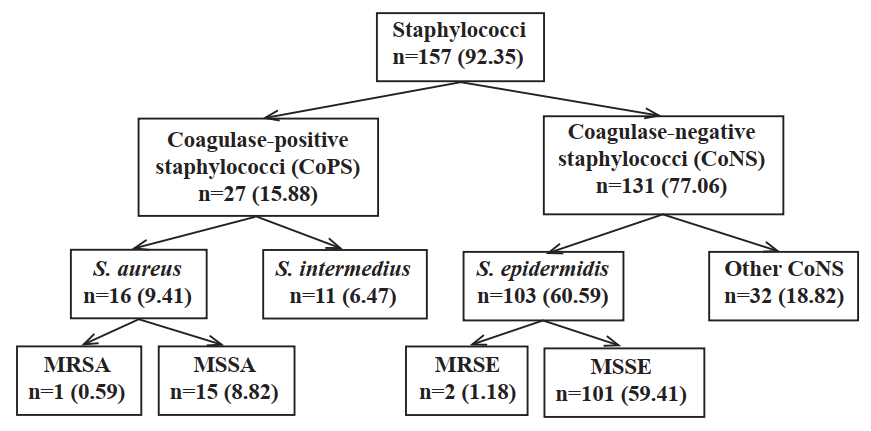
Results: Of 170 subjects, 157 (92.35%) revealed staphylococcal positive, yielding 161 staphylococcal isolates. The prevalence of MRSA, methicillin-resistant Staphylococcus epidermidis (MRSE), and methicillin-susceptible Staphylococcus aureus (MSSA) nasal carriage was 0.59, 1.18 and 8.82%, respectively. The MRSA isolate carried mecA revealing SCCmec type II. The MSSA isolates indicated low resistance to tetracycline (13.3%), whereas the MRSA isolate resisted ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin and tetracycline. Using multiple logistic regression analysis, a significant risk factor for S. aureus nasal carriage was utensil sharing (adjusted odds ratio=4.41; 95% CI=1.33-14.61).
Conclusion: Healthcare-associated MRSA existed among privates of the Medical Private Company. An associated risk factor for acquiring S. aureus was utensil sharing which could be used to help improve prevention and control management among privates.
Downloads
Metrics
References
Euzéby JP. List of bacterial names with standing in nomenclature: a folder available on the internet. Int J Syst Bacteriol 1997; 47: 590-92. https://doi.org/10.1099/00207713-47-2-590 PMid:9103655
NCBI Taxonomy browser. Staphylococcus. https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=1279&lvl=3&p=has_linkout&p=blast_url&p=genome_blast&lin=f&keep=1&srchmode=1&unlock. Accessed 31 March 2021.
Chessa D, Ganau G, Mazzarello V. An overview of Staphylococcus epidermidis and Staphylococcus aureus with a focus on developing countries. J Infect Dev Ctries 2015; 9: 547-50. https://doi.org/10.3855/jidc.6923 PMid:26142662
Janda WM, Ristow K, Novak D. Evaluation of RapiDEC Staph for Identification of Staphylococcus aureus, Staphylococcus epidermidis, and Staphylococcus saprophyticus. J Clin Microbiol 1994; 32: 2056-59. https://doi.org/10.1128/jcm.32.9.2056-2059.1994 PMid:7814525 PMCid:PMC263941
Szemraj M, Grazul M, Balcerczak E, Szewczyk1 EM. Staphylococcal species less frequently isolated from human clinical specimens - are they a threat to hospital patients? BMC Infect Dis 2020; 20: 128. https://doi.org/10.1186/s12879-020-4841-2 PMid:32046678 PMCid:PMC7014773
Backx M, Healy B. Serious staphylococcal infections. Clin Med 2008; 8: 535-38. https://doi.org/10.7861/clinmedicine.8-5-535 PMid:18975490 PMCid:PMC4953940
Von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344: 11-6. https://doi.org/10.1056/NEJM200101043440102 PMid:11136954
Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009; 7: 629-41. https://doi.org/10.1038/nrmicro2200 PMid:19680247 PMCid:PMC2871281
Barber M. Methicillin-resistant staphylococci. J Clin Pathol 1961; 14: 385-93. https://doi.org/10.1136/jcp.14.4.385 PMid:13686776 PMCid:PMC480239
Chiang CH, Pan SC, Yang TS, Matsuda K, Kim H, Choi Y, et al., Healthcare-associated infections in intensive care units in Taiwan, South Korea, and Japan: Recent trends based on national surveillance reports. Antimicrob Resist Infect Control 2018; 7: 129. https://doi.org/10.1186/s13756-018-0422-1 PMid:30455867 PMCid:PMC6223041
Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. Methicillin-resistant Staphylococcus aureus: The superbug. Int J Infect Dis 2010; 14(Suppl4): 7-11. https://doi.org/10.1016/j.ijid.2010.05.003 PMid:20851011
Polyzou A, Slavakis A, Pournaras S, Maniatis AN, Sofianou D, Tsakris A Predominance of a methicillin-resistant Staphylococcus aureus clone susceptible to erythromycin and several other non-β-lactam antibiotics in a Greek hospital. J Antimicrob Chemother 2001; 48: 231-34. https://doi.org/10.1093/jac/48.2.231 PMid:11481293
Benrabia I, Hamdi TM, Shehata AA, Neubauer H, Wareth G. Methicillin-resistant Staphylococcus aureus (MRSA) in poultry species in Algeria: Long-term study on prevalence and antimicrobial resistance. Vet Sci 2020; 7: 54. https://doi.org/10.3390/vetsci7020054 PMid:32349228 PMCid:PMC7356745
Malachowa N, Deleo FR. Mobile genetic elements of Staphylococcus aureus. Cell Mol Life Sci 2010; 67: 3057-71. https://doi.org/10.1007/s00018-010-0389-4 PMid:20668911 PMCid:PMC2929429
Yamaguchi T, Ono D, Sato A. Staphylococcal Cassette Chromosome mec (SCCmec) analysis of MRSA. Methods Mol Biol 2020; 2069: 59-78. https://doi.org/10.1007/978-1-4939-9849-4_4 PMid:31523765
Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, et al. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother 2006; 50: 1001-12. https://doi.org/10.1128/AAC.50.3.1001-1012.2006 PMid:16495263 PMCid:PMC1426434
Mollema FP, Richardus JH, Behrendt M, Vaessen N, Lodder W, Hendriks W, et al., Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 2010; 48: 202-07. https://doi.org/10.1128/JCM.01499-09 PMid:19923490 PMCid:PMC2812253
Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al., Communityacquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003; 9: 978-84. https://doi.org/10.3201/eid0908.030089 PMid:12967497 PMCid:PMC3020611
Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, et al., Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2001; 45: 1323-36. https://doi.org/10.1128/AAC.45.5.1323-1336.2001 PMid:11302791 PMCid:PMC90469
Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, et al., Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 2002 46: 1147-52. https://doi.org/10.1128/AAC.46.4.1147-1152.2002 PMid:11897611 PMCid:PMC127097
Aswapokee N, Chokecharoenrat S, Aswapokee P, Khongsamran S, Trisanananda M. Prevalence of methicillin-resistant staphylococci in a university hospital. J Med Assoc Thai 1982; 65: 28-32.
Jamulitrat S, Thongpiyapoom S, Varindsathien P, Ngo U, Promplook S. An outbreak of methicillin-resistant Staphylococcus aureus in a university hospital. J Infect Dis Antimicrob Agents 1988; 5: 103-10.
Al-Dahbi and Al-Mathkhury. Distribution of methicillin-resistant Staphylococcus aureus in Iraqi patients and healthcare workers. Iraqi J Science 2013; 54: 293-300.
Dulon M, Peters C, Schablon A, Nienhaus A. MRSA carriage among healthcare workers in non-outbreak settings in Europe and the United States: A systematic review. BMC Infect Dis 2014; 14: 363. https://doi.org/10.1186/1471-2334-14-363 PMid:24996225 PMCid:PMC4094410
Kitti T, Boonyonying K, Sitthisak S. Prevalence of methicillin-resistant Staphylococcus aureus among university students in Thailand. Southeast Asian J Trop Med Public Health 2011; 42: 1498-504.
Imwattana K, Bhumiwat P, Vorathongchai T, Kongurai W, Kiratisin P. High prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among Thai medical students. J Med Assoc Thai 2019; 102: 489-92.
Lulitanond A, Engchanil C, Chaimanee P, Vorachit M, Ito T, Hiramatsu K. The first vancomycin-intermediateStaphylococcus aureus strains isolated from patients in Thailand. J Clin Microbiol 2009; 47: 2311-16. https://doi.org/10.1128/JCM.01749-08 PMid:19403764 PMCid:PMC2708526
Phongsamart W, Srifeungfung S, Tiensasitorn C, Vanprapar N, Chearskul S, Chokephaibulkit K. The first pediatric case of Staphylococcus aureus with heterogenous resistant to vancomycin endocarditis in Thailand. J Med Assoc Thai 2005; 88(Suppl8): 264-68.
Yamane T. Statistics: An introductory analysis (2nd ed). New York: Harper and Row; 1967.
McClure JA, Conly JM, Lau V, Elsayed S, Louie T, Hutchins W, et al., Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from resistant staphylococci. J Clin Microbiol 2006; 44: 1141-44. https://doi.org/10.1128/JCM.44.3.1141-1144.2006 PMid:16517915 PMCid:PMC1393128
Chatreewattanakul T, Thunyaharn S, Samosornsuk W, Visawapoka U, Samosornsuk S. SCCmec type and antimicrobial susceptibility pattern of MRSA isolated from blood stream infection in Phramongkutklao Hospital. Arch AHS 2015; 27(2): 152-61.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty second informational supplement 2012; 32: M100-S22.
Blok HE, Troelstra A, Kamp-Hopmans TE, Gigengack-Baars AC, Vandenbroucke-Grauls CM, Weersink AJ, et al., Role of healthcare workers in outbreaks of methicillin-resistant Staphylococcus aureus: A 10-year evaluation from a Dutch university hospital. Infect Control Hosp Epidemiol 2003; 24: 679-85. https://doi.org/10.1086/502275 PMid:14510251
Giri S, Ghimire A, Mishra A, Acharya K, Kuikel S, Tiwari A, et al. Prevalence of methicillin-resistant Staphylococcus aureus carriage among healthcare workers in South Asia in non-outbreak settings: A systematic review and meta-analysis. Am J Infect Control 2022; 10: S0196-6553(22)00474-6. https://doi.org/10.1016/j.ajic.2022.06.001 PMid:35697125
Treesirichod A, Hantagool S, Prommalikit O. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus among medical students at the HRH Princess Maha Chakri Sirindhorn Medical Center, Thailand: A cross-sectional study. J Infect Public Health 2013; 6: 196-201. https://doi.org/10.1016/j.jiph.2012.12.004 PMid:23668464
Turabelidze G, Lin M, Wolkoff B, Dodson D, Gladbach S, Zhu BP. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006; 12: 422-27. https://doi.org/10.3201/eid1203.050625 PMid:16704779 PMCid:PMC3291434
Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of premethicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother 1999; 43: 1449-58. https://doi.org/10.1128/AAC.43.6.1449 PMid:10348769 PMCid:PMC89295
Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V Staphylococcal Cassette Chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrobial Agents Chemother 2004; 48: 2637-51. https://doi.org/10.1128/AAC.48.7.2637-2651.2004 PMid:15215121 PMCid:PMC434217
Masmalai T, Kasitibodinchai M, Chanawong A, Sribenjalux P, Sinlapasorn S, Sungkeeree S, et al. Survey of methicillin-resistant Staphylococcus aureus among students in the Faculty of Associated Medical Sciences, Khon Kaen University. J Med Tech Phy Ther 2016; 28: 1-8.
Bloemendaal AL, Brouwer EC, Fluit AC. Methicillin resistance trancsfer from Staphylococcus epidermidis to methicillin-susceptible Staphylococcus aureus in a patient during antibiotic therapy. PLoS One 2010; 5: e11841. https://doi.org/10.1371/journal.pone.0011841 PMid:20686601 PMCid:PMC2912275
Downloads
Published
How to Cite
Issue
Section
License
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







