SENSITIVITY AND SPECIFICITY OF INSM1 IN DIFFERENTIATING NEUROENDOCRINE CARCINOMA OF THE HEAD AND NECK FROM NONKERATINIZING NASOSPHARYNGEAL CARCINOMA AND P16-POSITIVE OROPHARYNGEAL SQUAMOUS CELL CARCINOMA
DOI:
https://doi.org/10.55374/jseamed.v9.243Keywords:
INSM1, neuroendocrine carcinoma, head and neck, specificity, sensitivity, interpretationAbstract
Background: Neuroendocrine carcinomas (NECs) rarely arise in the head and neck region. Their diagnosis presents challenges due to morphological overlap with other entities, particularly nonkeratinizing nasopharyngeal carcinomas (NK-NPC) and p16-positive oropharyngeal squamous cell carcinomas (p16-positive OPSCC), both of which are prevalent in Thailand. Insulinoma-associated protein 1 (INSM1) is a relatively new marker that has demonstrated favorable sensitivity and specificity in various organs. However, despite its promising potential, there is a paucity of studies investigating its utility in the head and neck region compared to other anatomical sites, especially in Thailand.
Objectives: This study aimed to evaluate the diagnostic performance of INSM1 in distinguishing NECs of the head and neck region from NK-NPC and p16-positive OPSCC by comparing its sensitivity and specificity with classic neuroendocrine markers, chromogranin A (CGA) and synaptophysin (SYN).
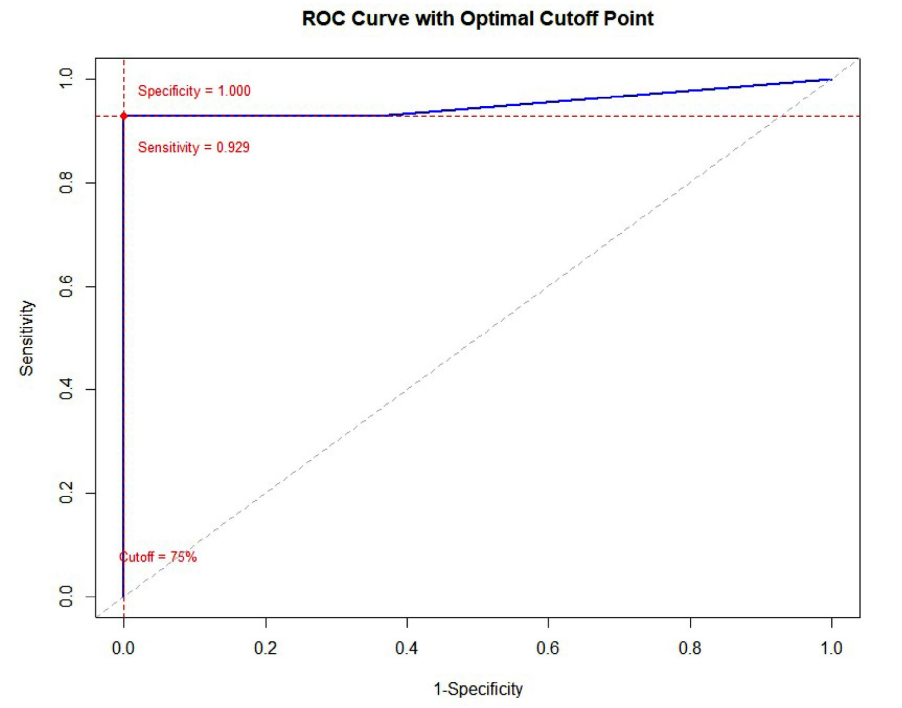
Methods: This retrospective cohort study analyzed 14 samples of NEC and 109 samples, comprising 93 NK-NPC and 16 p16-positive OPSCC cases. Immunohistochemical (IHC) staining for INSM1, CGA, and SYN was performed on all cases. Receiver Operating Characteristic (ROC) curve analysis was utilized to determine the optimal cutoff point for INSM1 positivity, maximizing both sensitivity and specificity.
Results: INSM1 demonstrated an overall sensitivity of 92.9% for head and neck NECs, comparable to SYN (100.0%, p = 0.001) but significantly higher than CGA (78.6%, p = 0.006). All three markers (INSM1, CGA, and SYN) achieved 100.0% specificity in differentiating NECs from NK-NPC and p16-positive OPSCC. ROC analysis determined an optimal cutoff of 75% tumor cell positivity for INSM1, with a Youden’s index of 0.93 and an Area Under the Curve (AUC) of 0.952, indicating excellent diagnostic accuracy. Notably, one case of Epstein-Barr virus (EBV)-positive NK-NPC exhibited INSM1 positivity in 40% of tumor cells with moderate to strong intensity.
Conclusion: INSM1 exhibits good sensitivity and excellent specificity for head and neck NECs, comparable to or surpassing those of CGA and SYN, respectively. While its high specificity is valuable, the observed positivity in a subset of NK-NPC cases, even below the optimal cutoff, suggests that INSM1 should not be used as a standalone diagnostic marker for NECs. Caution is advised when interpreting INSM1 staining in less than 75% of tumor cells, as this may reduce the reliability of a positive finding. A comprehensive panel that includes classic neuroendocrine markers and, where appropriate, EBER in situ hybridization remains crucial for accurate diagnosis.
Downloads
References
Ohmoto A, Sato Y, Asaka R, Fukuda N, Wang X, Urasaki T, et al. Clinicopathological and genomic features in patients with head and neck neuroendocrine carcinoma. Mod Pathol 2021; 34: 1979-89. DOI: https://doi.org/10.1038/s41379-021-00869-9
Alos L, Hakim S, Larque AB, Rodriguez-Carunchio L, Caballero M, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch 2016; 469: 277-84. DOI: https://doi.org/10.1007/s00428-016-1982-1
Su Z, Yan H, Zou G. EBV positive large cell and small cell neuroendocrine carcinoma of the nasopharynx: a case report and review of the literature. Oncol Lett 2022; 24: 1. DOI: https://doi.org/10.3892/ol.2022.13584
Chen Y, Zhou N, Huang C, He X, Wang X, Tang H, et al. EBV-positive small cell neuroendocrine carcinoma of nasopharynx as a probably unique subtype of neuroendocrine carcinoma: a clinicopathologic study of three cases and literature review. Diagn Pathol 2024; 19: 1. DOI: https://doi.org/10.1186/s13000-024-01526-w
Petersson F. Nonkeratinizing nasopharyngeal carcinoma with adenomatous differentiation. Head Neck Pathol 2020; 14: 195-8. DOI: https://doi.org/10.1007/s12105-018-0991-6
Al Masalmeh N, Kukreja G, Zaiem F, Raza SN, Kim H, Nagasaka M, et al. p16 positive oropharyngeal small cell cancer: a case report. Oral Oncol 2021; 121: 105391 DOI: https://doi.org/10.1016/j.oraloncology.2021.105391
Uhlig R, Dum D, Gorbokon N, Menz A, Büscheck F, Luebke AM, et al. Synaptophysin and chromogranin A expression analysis in human tumors. Mol Cell Endocrinol. 2022; 555: 111726. DOI: https://doi.org/10.1016/j.mce.2022.111726
Tomita T. Significance of chromogranin A and synaptophysin in pancreatic neuroendocrine tumors. Bosn J Basic Med Sci 2020; 20: 336-46. DOI: https://doi.org/10.17305/bjbms.2020.4632
Rinaldo A, Devaney KO, Ferlito A. Immunohistochemical studies in support of a diagnosis of small cell neuroendocrine carcinoma of the larynx. Acta Otolaryngol 2004; 124: 638-41. DOI: https://doi.org/10.1080/00016480410016540
Rooper LM, Bishop JA, Westra WH. INSM1 is a sensitive and specific marker of neuroendocrine differentiation in head and neck tumors. Am J Surg Pathol 2018; 42: 665-71. DOI: https://doi.org/10.1097/PAS.0000000000001037
Yuan C, Jiao F, Zhai C, Zhang J, Wang S, Zhu L. Application of INSM1 in diagnosis and grading of laryngeal neuroendocrine carcinoma. Laryngoscope 2021; 131: E2662-8. DOI: https://doi.org/10.1002/lary.29554
Mukhopadhyay S, Dermawan JK, Lanigan CP, Farver CF. Insulinoma-associatedprotein1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod Pathol 2019; 32: 100-9. DOI: https://doi.org/10.1038/s41379-018-0122-7
Kriegsmann K, Zgorzelski C, Kazdal D, Cremer M, Muley T, Winter H, et al. Insulinomaassociated protein 1 (INSM1) in thoracic tumors is less sensitive but more specific compared with synaptophysin, chromogranin A, and CD56. Appl Immunohistochem Mol Morphol 2020; 28: 237-42. DOI: https://doi.org/10.1097/PAI.0000000000000715
Sakakibara R, Kobayashi M, Takahashi N, Inamura K, Ninomiya H, Wakejima R, et al. Insulinoma-associated protein 1 (INSM1) is a better marker for the diagnosis and prognosis estimation of small cell lung carcinoma than neuroendocrine phenotype markers such as chromogranin A, synaptophysin, and CD56. Am J Surg Pathol 2020; 44: 757-64. DOI: https://doi.org/10.1097/PAS.0000000000001444
Rocha R, Henrique R. Insulinoma-associated protein 1 (INSM1): diagnostic, prognostic, and therapeutic use in small cell lung cancer. J Mol Pathol 2022; 3: 140-69. DOI: https://doi.org/10.3390/jmp3030013
Rooper LM, Sharma R, Li QK, Illei PB, Westra WH. INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin, and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol 2017; 41: 1561-9. DOI: https://doi.org/10.1097/PAS.0000000000000916
Kim IE Jr, Amin A, Wang LJ, Cheng L, Perrino CM. Insulinoma-associated protein 1 (INSM1) expression in small cell neuroendocrine carcinoma of the urinary tract. Appl Immunohistochem Mol Morphol 2020; 28: 687-93. DOI: https://doi.org/10.1097/PAI.0000000000000824
Lewis JS, Chernock RD, Bishop JA. Squamous and neuroendocrine specific immunohistochemical markers in head and neck squamous cell carcinoma: a tissue microarray study. Head Neck Pathol 2018; 12: 62-70. DOI: https://doi.org/10.1007/s12105-017-0825-y
Zhao Y, Qiu Z, Ye S. Identifying immune cell infiltration and diagnostic biomarkers for nasopharyngeal carcinoma through bioinformatic analysis. Res Square 2024. DOI: https://doi.org/10.21203/rs.3.rs-3816483/v1
Daniel WW, Cross CL. Biostatistics: a foundation for analysis in the health sciences. 10th ed. Hoboken, NJ: Wiley; 2013.
Möller K, Gorbokon N, Hube-Magg C, Fraune C, Lennartz M, Blessin NC, et al. Comparison of INSM1 immunostaining with established neuroendocrine markers synaptophysin and chromogranin A in over 14,000 neuroendocrine and non-neuroendocrine tumors. Am J Clin Pathol 2023; 160 (Suppl 1): S36-7. DOI: https://doi.org/10.1093/ajcp/aqad150.081
Hajra S, Kumar A, Sundriyal D, Chandra H, Balasubramanian P. A rare case report of nasopharyngeal carcinoma with multi-organ metastasis, clinico-radiologically mimicking lymphoma. Hematol Transfus Cell Ther 2024; 46 (Suppl 6): S389-S92. DOI: https://doi.org/10.1016/j.htct.2023.03.024
Hwang TZ, Jin YT, Tsai ST. EBER in situ hybridization differentiates carcinomas originating from the sinonasal region and the nasopharynx. Anticancer Res 1998; 18: 4581-4.
Murono S, Yoshizaki T, Tanaka S, Takeshita H, Park C, Furukawa M. Detection of Epstein-Barr virus in nasopharyngeal carcinoma by in situ hybridization and polymerase chain reaction. Laryngoscope 1997; 107: 523-6. DOI: https://doi.org/10.1097/00005537-199704000-00017
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 Journal of Southeast Asian Medical Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







